The dry reforming of methane to convert CO2 and methane into synthesis gas (hydrogen and carbon monoxide) is another process which is able to produce value-added products from CO2. The Particles and Catalysis Research Group has focussed in this area of research and has developed key specialisations. Both companies Linde and Sasol have shown this process to be viable at an industrial scale.
CO2 + CH4 → 2H2 + 2CO
The Particles and Catalysis Research Group’s work on dry reforming of methane reaction has focussed on developing active and stable catalysts which are based on cheap active metals (Ni and Co) and supports (SiO2, Al2O3). The team has found the key to catalyst performance for Ni-based catalysts is the prevention of carbon formation. By combining Ni with Co on an Al2O3 support, PartCat was able to achieve a stable CH4 conversion of 50-90% at 600 800°C. A similar effect was found by adding La to the Al2O3 support. PartCat is working on improving the performance of SiO2-supported Ni catalysts. Modifying the support with CeZrO2 was found to enhance catalyst stability.
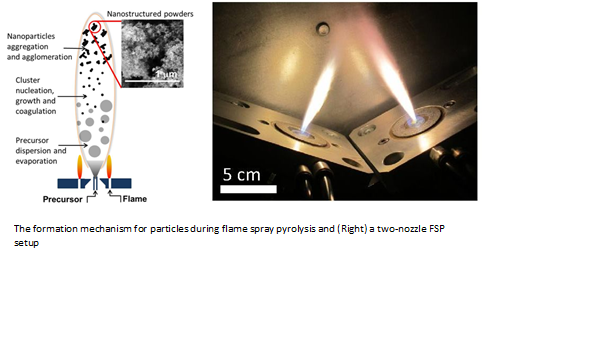
A unique area of research which the Particles and Catalysis Research Group has applied to the dry reforming of methane work is to incorporate the flame spray pyrolysis process (shown in Figure 2). The flame spray pyrolysis is a rapid and single-step method for producing high surface area metal oxides which can be readily scalable. The team has demonstrated that highly active dry reforming of methane catalysts based on Co and an Al2O3 support can be synthesised via flame spray pyrolysis method. When doped with a small amount of La, the Co/Al2O3 materials are able to achieve stable conversion of 85-90% at an operating temperature of 700°C. To reach 700°C, this can be achieved using solar collector, which would also in turn reduce the energy required for heating.